CLINIVI

At Clinivi, we provide a comprehensive, end-to-end service to take your vision — whether it’s a new clinic, a surgical centre, or a hospital — from concept to compliant, operational reality. Our consultants possess extensive expertise in healthcare law, governance, and regulatory compliance, with proven experience supporting new services across both private and NHS sectors. From planning and design through to CQC registration, policies, staffing, and patient pathways, we ensure your service meets all legislative and regulatory requirements under the Health and Social Care Act 2008 (Regulated Activities) Regulations 2014, the Health and Safety at Work etc. Act 1974, and COSHH Regulations 2002. We tailor our involvement to your needs — providing full-scale project delivery or focused support in specific areas such as infection control design, governance frameworks, or staff compliance training. Every plan is bespoke and co-created with you to ensure sustainability, safety, and CQC readiness.

Navigating the Care Quality Commission (CQC) process can be complex.
We provide expert assistance with every aspect of registration and ongoing compliance:
• Completion of provider and manager application forms
• Guidance on regulated activities, fit and proper person requirements (Regulation 5), and duty of candour (Regulation 20)
• Mock interviews and coaching for nominated individuals and registered managers
We also provide education sessions to help your team understand CQC’s Key Lines of Enquiry (KLOEs), fundamental standards, and best practice in demonstrating safe, effective, caring, responsive, and well-led services.
Our goal is to help you not just meet compliance standards — but achieve a rating of Outstanding.
Governance Support
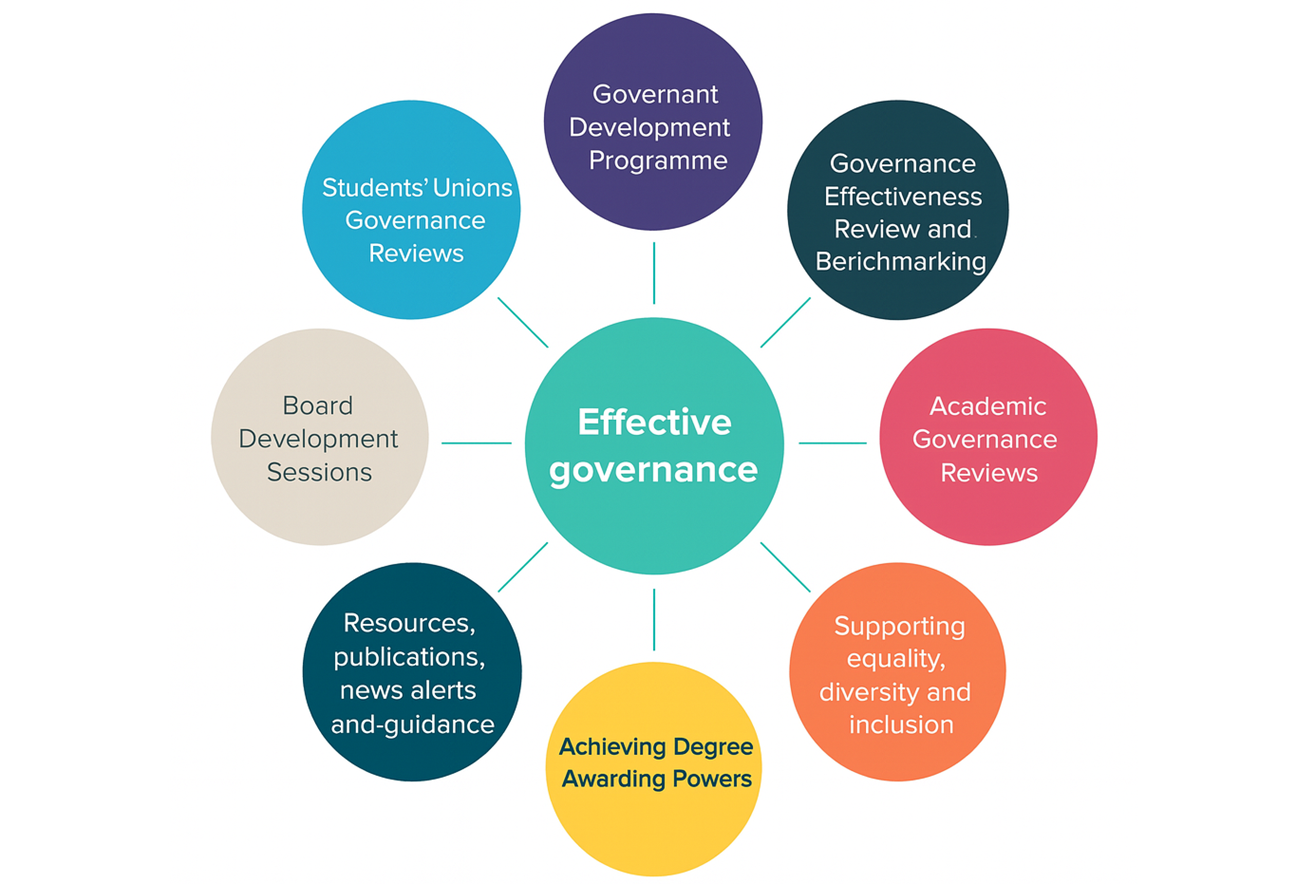
A compliant and sustainable healthcare business relies on strong governance. We help you design and embed governance frameworks aligned with NHS England governance principles, CQC Regulation 17 (Good Governance), and ISO 9001:2015 Quality Management Systems.
• Risk management systems and registers
• Incident reporting and learning culture frameworks
• Complaint handling and patient feedback analysis
• Clinical audit structures
• Policy and procedural oversight
• Quality improvement cycles and safety committees
We ensure your governance model evolves alongside your organisation — adaptable, transparent, and regulatory-ready.
Training and Support

Competence and continuous learning are legal and professional imperatives.
We offer tailored training to meet the requirements of CQC Regulation 18 (Staffing),
Health and Safety at Work Regulations 1999, and Infection Prevention and Control Standards.
Programmes include:
• Mandatory and statutory training (fire safety, COSHH, manual handling, safeguarding, etc.)
• Clinical competencies such as aseptic technique, wound care, drain management, and suture removal
• Audit methodology and governance training
• Leadership and compliance coaching for registered managers
We ensure your staff are not only compliant — but confident.

Our full-service CQC registration package includes:
• Completion of all CQC forms and supporting documents
• Bespoke policies and operational templates
• Unlimited 1:1 coaching and mock interviews
• Site inspection readiness assessments
We ensure you are fully compliant under the Health and Social Care Act 2008,
with evidence portfolios and governance frameworks designed to meet inspection expectations.

We provide full IPC support in compliance with the Health and Social Care Act 2008:
Code of Practice on the prevention and control of infections, and HTM 03-01
(Specialised ventilation for healthcare premises).
Our team can assist with:
• Environmental audits and risk assessments
• Compliance with design standards for sinks, air handling, laminar flow, and UV sterilisation
• Training on aseptic technique, sepsis management, wound care, and clinical hygiene
• IPC lead mentoring and annual statement development
From the cleaner managing COSHH safely to the surgeon maintaining aseptic technique —
Clinivi ensures total infection control assurance.

We offer in-depth mock inspections replicating CQC methodology, assessing compliance across the five KLOEs.We identify gaps, recommend corrective actions, and coach your teams to handle inspection interviews confidently.Our aim: zero surprises on inspection day — and maximum preparedness to evidence excellence.

We provide HR support aligned with Employment Law, the Equality Act 2010, and ACAS Codes of Practice.
Our HR outreach services include:
• Safe recruitment under CQC Regulation 19 (Fit and Proper Persons: Employment)
• DBS compliance and Right to Work verification
• Staff handbooks, contracts, and disciplinary frameworks
• Workforce wellbeing and retention strategies
We help you foster a compliant, fair, and supportive working environment at every level.

Compliant complaint handling is essential under CQC Regulation 16 (Receiving and Acting on Complaints) and The Local Authority Social Services and NHS Complaints Regulations 2009.
When a complaint escalates beyond internal resolution, we offer Stage 3 independent adjudication — ensuring transparency, fairness, and learning.
Our experienced complaint investigators provide balanced reviews that protect your organisation’s integrity while maintaining patient trust.
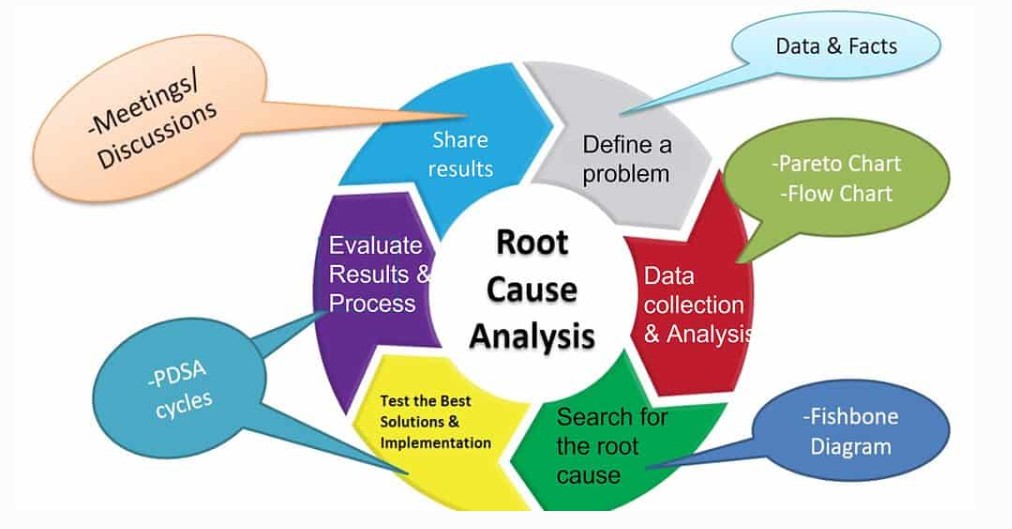
In line with Duty of Candour (Regulation 20) and NHS Serious Incident Framework, we conduct independent RCAs that prioritise learning over blame.
We help you identify causative factors, implement corrective actions, and embed safety learning across your workforce.

Offering endoscopy services requires strict adherence to Joint Advisory Group (JAG) Accreditation Standards.
We support you through the entire JAG accreditation process, ensuring compliance in clinical quality, safety, environment, decontamination, and patient experience.
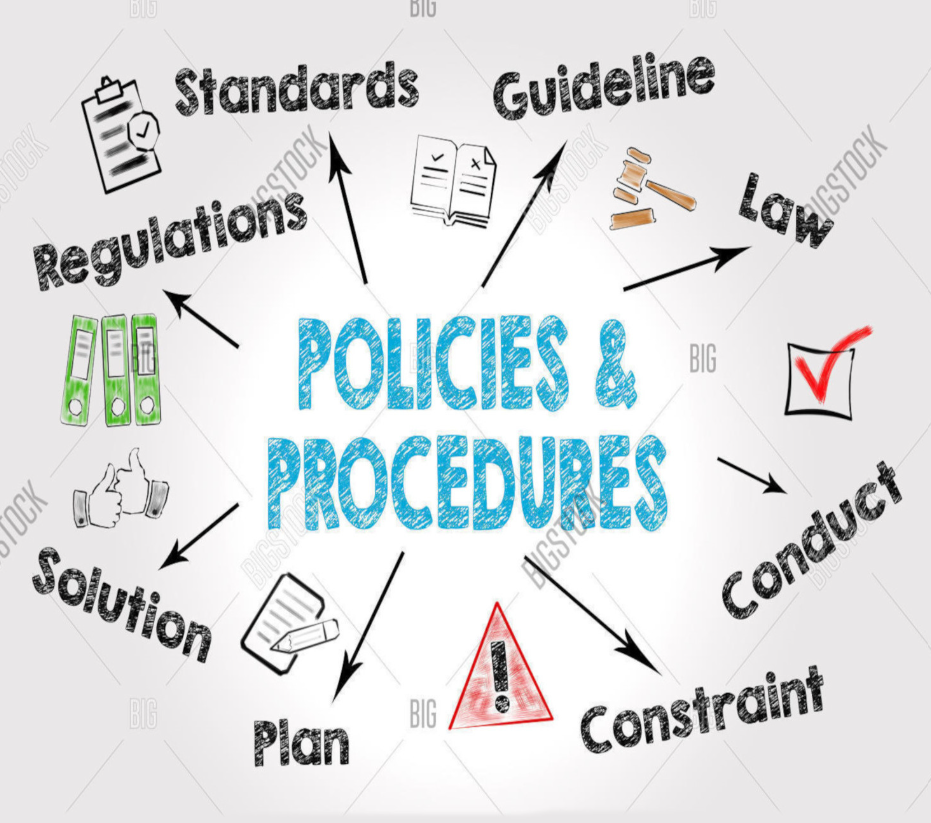
Healthcare compliance demands extensive and regularly updated documentation.
We help you develop, review, and maintain:
• Policies and Standard Operating Procedures (SOPs)
• Clinical and operational audits
• Risk assessments and COSHH documentation
• Daily checklists and record templates
All policies are aligned with CQC regulations, Health and Safety Executive (HSE) guidance,
NICE standards, and the Data Protection Act 2018 (GDPR).
We also monitor legislative changes to keep your documentation continuously compliant.

We design robust risk management systems aligned with ISO 31000 (Risk Management) and CQC Regulation 17 (Good Governance).
Our consultants assess clinical and non-clinical risks, create structured risk registers, and implement monitoring processes to reduce harm, support assurance, and satisfy regulators.
This integrated framework strengthens safety, accountability, and confidence at every operational level.